Return to Historical Geology Return to Physical Geology
Rocks can be dated using two techniques. One of these is relative dating through which comparative ages are indicated. One can establish this rock is older than that, using these techniques, but not how old either is. On the other hand, absolute dating techniques are used to establish an exact (more or less) age for when the rock was formed. Not all rocks can be dated this way. Refer back to your slides and the OER textbook for more information.
Every radioactive isotope has a unique half-life. (Refer back to the slides or the OER textbook for more information.) Knowing the half-life and establishing the percent of each part of the decay enables one to establish how long the elements within the rock have been in decay, or when the rock formed.
Let’s look at an example.
Tthe radioactive half-life for Uranium 235 (U-235) is 704,000,000 years. When the rock bearing U-235 is formed it has 100% U-235 isotopes. It takes 704 mya for half of the U-235 to turn into Lead 208 (Pb-208). In another 704 mya, or a total of 1408 mya, there will be 25% of the original U-235 and 75% will be Pb-208. This continues until the trace amounts of U-235 are negligible.
So, what does this mean for us? It means that knowing the half-life you can calculate the age of a rock or knowing the age you can calculate the half-life.
Example 1
A sample of rock contains 12.5% U-235 and 87.5% Pb-208. How old is the sample?
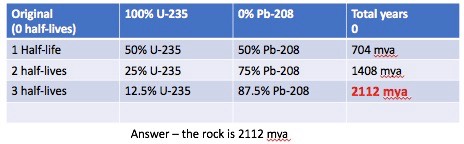
Example 2
How much of the original K-40 remains after 2 half-lives?
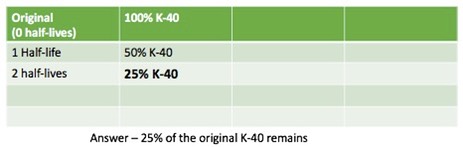
Example 3